

Feminine and masculine brains: from talents to pugnacity styles
The most common notion about gender hiatuses today is that cognitive differences between women and men are minimal and superfluous. Women’s entry into all spheres of professional activity has been so comprehensive and formidable that it is difficult to avoid that certainty. The prospect of strict equality for both genders in all aptitudes and activities is taken for granted, and the persistence of biases in preferences and interests which may derive from propensities rooted the biology of sexual differentiation is a ‘minor’ and neglected detail. However, that egalitarian prescription is centered around an error which arises from overlooking an arsenal of findings on the matter. In fact, there are prominent anatomical and physiological differences between women and men, just as there usually are in other mammals. There are also prominent differences in their affective and emotional reaction profiles, and in motor, sensory and cognitive domains. The distinctions between the sexes affect practically all the body’s systems and machinery, and they rely on intricate mechanisms. This is also true of the brain, which is the gland where the usual – and not always benign – conflicts of gender emerge.
However, it can still be said that women achievements in any area of education, as well as in hierarchies within businesses, corporations and institutions have been overwhelming in recent decades, and that the changes brought about by feminine power have been no less significant. Nevertheless, there are still some professional and recreational strongholds that are less conducive to the advancement of women. In scientific disciplines, for example, progress has been meteoric overall. In the USA, while less than 5% of doctorates in science and medicine in 1970 were awarded to women, in 2006, women took 51% of doctorates in biology, 76% in veterinary science, 67% in psychology and 50% in medicine (Ceci & Williams, 2010a, 2010b). This is a drastic turnaround, although these percentages fall short compared to Europe, where there has been a shift towards a feminine monopoly in these areas, with men being almost completely excluded. However, there is one exception: the rise of women has not been as forceful in disciplines with a heavy engineering or mathematical focus. In 2006, less than one third of these doctorates in the USA went to women: 29.6% in maths, 21.3% in computer sciences, 29% in physics and 20.2% in engineering. According to data from the top 100 universities in the USA, female success on the faculty-staff ladder in these disciplines varied from 8.8% in mechanical engineering to 15.8% in astronomy (Nelson & Brammer 2010), and when it came to professorships, the benchmark of 10% was barely met: 9.7% in chemistry, 7.1% in maths, 10.3% in computer sciences, 6.1% in physics, 7.3% in chemical engineering, 7.1% in electrical engineering, 7.3% in highway engineering, 4.4% in mechanical engineering and 8.7% in economics. The origin of this discrepancy has been hotly debated, and there have been well-known incidents such as the dismissal of Larry Summers – a prestigious economist and director of the National Economic Council during the Clinton and Obama administrations – as Harvard University’s president in 2006, for expressing ‘incorrect’ opinions on the subject.
The old dilemma of ‘natural’ genesis (distinct nervous and endocrine systems) versus the effects of biased ‘educational environments’ (upbringing according to the roles expected of girls and boys), has become entrenched at the heart of disputes over the question of the ceilings reached by men and women[1]. I am going to delve into this subject through these irritating and unclosed frontiers, while approaching the question from other directions also.
Male and female bodies: hormonal impregnation.
By contrast, no one disputes the origin of the wonderful morphological differentiation between men and women: the notable differences between the male chassis and frame, and female curves with the shape of their more distinctive bodily embellishments. Here, there is no conflict, despite the complication of the stages of intersex and radical re-tuning of bodywork often required by transexuals who feel intimately uncomfortable with the male or female mould in which they arrived, spontaneously, upon maturing. Through corrective and reconstructive surgery, combined with hormonal treatment, they can move from one body shape to another, with undeniable discomfort but often towards an impeccable sculptural definition.
There is no question about that: the body can be moulded and imbalances resolved through morphological (biophysical) and molecular (biochemical, hormonal) changes. There is no doubt, hesitation or mystification around the role of sex hormones in the formation of the ‘athletic’ masculine prototype and the curvaceous female figure. It is accepted, without question, that besides the distinctive organisation of the genitals, the distribution of bones, muscles and fatty tissue, the particular distribution of hair, the qualities of the skin, and even the different tonal modalities in the voice when speaking or singing, this is the result of hormones acting on target tissues at specific stages of growth. It is worth highlighting here that the different registers of the male and female voice produce different sound typologies for both genders in ‘bel canto’ and in any musical environment, without that causing conflicts between acclaimed, commendable or amateur singers. That is to say that there are no known claims or measures to promote gender parity between baritone, bass, mezzo and sopranos, although these and other vocal specialisations depend on subtle and distinct neuromotor regulation mechanisms.
Nor are there any serious aspirations to achieve gender parity with regard to pleasurable sexual reactions. In fact, the enjoyment of different reflexes of the human male and female response during the excitement of foreplay and copulation is usually cause for joyful celebration. These glaring singularities (penile erection and vaginal lubrication, to give the most obvious examples) obey intricate neuromotor circuits with their own regulatory, spinal and encephalic specialisations (Tobeña 2006, Pfaff 2011). A distinctive specialisation by sex that also supports the cerebral modulation of pleasure, excitement or amorous satiety (Giorgiadis et al, 2012). This need to emphasise, wherever possible, the differences in the rhythms and depth of sexual enjoyment between genders also extends to the range of preferences for subjects of erotic interest, and that requires much ‘higher’ neural regulation, so to speak; distinct from automatic or ‘instinctive’ mediation.
Disparate brains: from moulds to talents.
Nevertheless, the most decisive juncture in the debates over gender hiatuses is usually over the more ‘sophisticated’ encephalon, because everyone understands that this is where the nuclear vectors of ingenuity, judgement and temperament reside (Kimura 2000, Shah et al, 2004, Hyde and Metz 2009). Given that there are well-founded suspicions and an abundance of data regarding the differences in talent and character of men and women, there is some impatience to link them to distinctions in the organisation and functions of the cortical and subcortical areas. The first attempts at precise measurement in a set of autopsied, normal brains immediately corroborated that female cerebral volume is smaller than in the male, both in absolute terms and in relative terms based on height and body size. This volumetric difference varies between 130 and 200 cc on average, in favour of men. The difference is not reflected in mental acuity as there are no discernible differences between genders in measurements of overall intelligence. Where there are, they are slightly in favour of females. In simple terms, human specimens of both sexes achieve equivalent cognitive acuity with different sized brains, so the differences in overall volume do not explain the distinctions in talents and temperament.
At the start of this century, there was a proliferation of comparative maps based on a set of male and female brains scanned using magnetic resonance imaging (MRI). In a study of 40 normal male brains and 40 normal female brains from people between 18 and 45 years old, neuroradiologists in Pennsylvania led by Rubén Gur (1999) confirmed these overall differences by using automated processes to segment grey matter, white matter and cerebrospinal fluid. They also corroborated previous findings that indicated that in the female encephalon, the relative percentage of grey matter is larger, while in men, white matter and cerebrospinal fluid compartments are predominant. They also discerned the existence of asymmetries in the grey and white encephalic segments between the right and left hemispheres – substantial inter-hemispheric discrepancies that are not seen in women. The volumes of grey and white matter were linked to cognitive performance in a package of neuropsychological tests, confirming the positive relationship between the size of the several parts of the cranial gland, and acuity and precision in mental work. These differences in volumetric percentages and asymmetrical distribution of neural tissue could determine the cognitive differences between the two genders. The researchers dared to conjecture that, as grey matter is made up of neuronal cell bodies and dendrite endings, while white matter is formed of axonal extensions, the female encephalon might compensate for its smaller size and remote inter-connectivity with increased computational ability in denser, better packaged circuits.
Goldstein y col. (2001) compared the regional volumes in 27 male brains and 21 female brains of the same age, manual laterality, intelligence, education and socio-economic level, taking the total cerebral volume as a reference. They detected differences between genders in a number of cortical and subcortical areas, with a female pattern showing a larger relative volume in different zones of the neocortex, particularly in the frontal and cingulate regions. The volumes in men were larger in the amygdala, hypothalamus and the frontal medial cortex, confirming a number of previous findings in anatomical, post-mortem studies. These differences were also linked to the different regional concentrations of sex steroid receptors, according to estimations made in mammals (from rodents to macaques), which indirectly corroborates the influence of these hormones on the differences in the encephalic structure in both sexes.
Table 1. Volumes (cc) of the cerebral segments by region with the proportion of G/W (grey matter/white matter), the zonal differences between genders, the magnitude of the effect and the statistical significance of the contrasts. (taken from Allen et al, 2003)
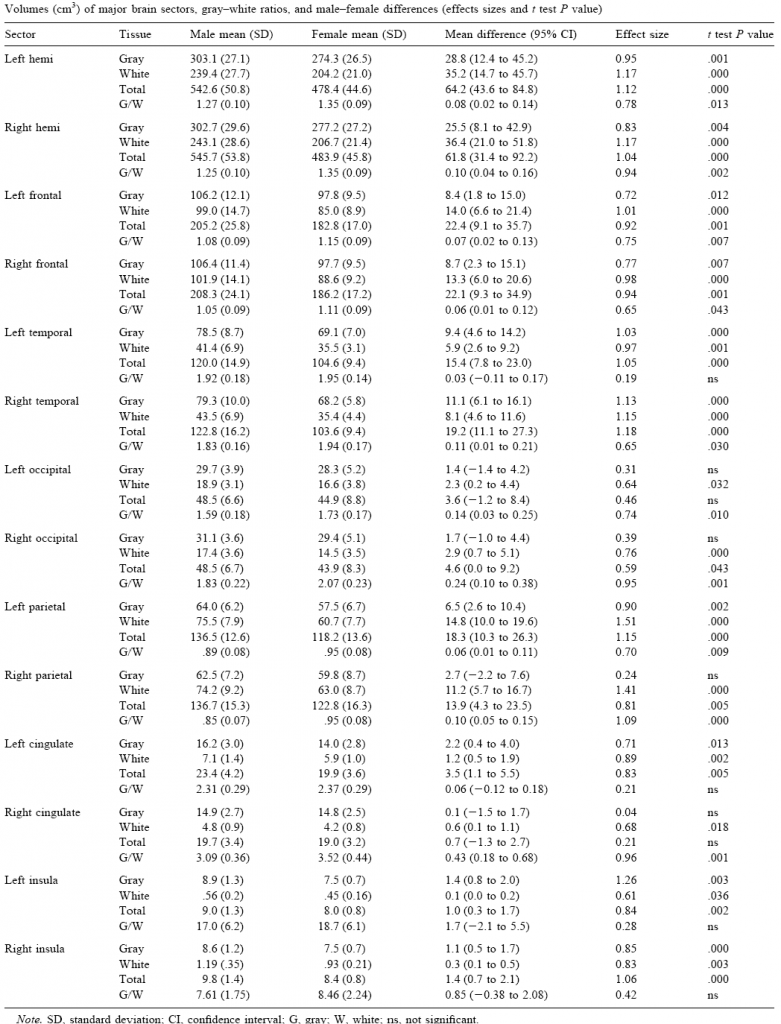
Table 1 contains the values of comparisons made with an MRI scan using automated processes to segment tissue and zones in 23 normal men and 23 normal women, all adult and right-handed, done by Hanna Damasio’s team in Iowa (Allen et al, 2003). In all of the encephalic structures, the overall male volumes and grey and white matter volumes were larger than those in females, although the G/W (grey matter/white matter) proportion was almost always higher in females. The distance between the sexes was higher for white matter than for grey matter, and it must be said that a lot of the variations in the proportion between one segment and another were derived from the presence of less white matter in females. This overall pattern was repeated in the majority of cortical regions with some slight exceptions. Despite the considerable consistency with previous findings, whether using automated segmentation methodologies or post-mortem measurements, the authors were cautious in linking these sexual dimorphisms with distinctive patterns in cognitive performance between genders.
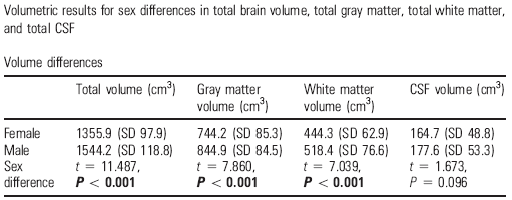
In addition to these zonal contrasts in tissue segments and encephalic fluid, they also made overall approximations by studying the cortical thickness in normal samples across a wide range of ages. A study by a group of UCLA neuroradiologists, led by Arthur Toga, examined this in 176 subjects between the ages of 7 and 87, and compared regional and overall differences in cerebral volume (Sowell et al, 2007; Table 2). Fine mapping of sex differences in cortical thickness revealed that women had a larger neocortex in the inferior parietal and posterior temporal regions. In these regions, the grey matter border was 0.45 mm and was thicker in women than in men. Comparison of an adult sub-sample of 18 men and 18 women of similar ages corroborated these findings independently of overall volumes and body size. This female superiority in terms of temporo-parietal grey matter seems to be consistent, and not dependent on age. Men were found to have greater volumes in the pre-frontal and occipital regions. Additional studies also corroborated that the female brain compensates for its smaller size with more complex folding of the cortext.
Table 2. Differences between the sexes in the total cerebral volume, grey matter, total white matter and cerebrospinal fluid in men (N=90) and women (N=86) in the UCLA sample of 176 subjects (taken from Sowell et al, 2007)
Comparisons throughout encephalic development and maturity have culminated, carefully, in a number of studies dedicated to children and adolescents (Lenrot et al, 2007), and the pace of differential ageing according to gender has also been compared. With regard to potential links with IQ measurements, it is important to refer to a pioneering study by UCLA (Haier et al, 2005) that used 48 normal subjects from two samples – one of young adults (average age = 27+/-6 years) and another of people at an advanced stage of maturity (average age = 59 +/-16 years). The links between the structural variations in the brains of men and women were analysed alongside the IQ scores. Most of these relationships were positive but there were notable differences between both sexes. The strongest and broadest associations between grey matter and overall IQ in men were found in both frontal lobes (BA 8, 9) and in the left parietal lobe (BA 39, 40) – the Wernicke zones. By contrast, in women, these links were limited to the right frontal lobe (BA10) and particularly the Broca area (BA 44, 45), although they were not as broad as in men. However, there was much more co-variation between white matter and IQ in women than in men in several cortical zones, although with frontal predominance, whereas in grey matter it was the exact opposite: greater associations in men than in women. The links with manipulative and verbal IQ measurements were tenuous. As a result, the conclusion is again that women and men achieve similar scores for overall cognitive acuity with quite different brains that have a different regional contribution from zones and compartments. This supports the notion that there are distinguishable neural bases for cognitive talents (Luders et al. 2004, Clayden et al. 2012; Escorial et al 2015).
The correspondence between sex differences in the organisation of encephalic zones and different cognitive abilities in both genders emerges regularly, albeit moderately. A meticulous study of around 100 Madrid university students (Burgaleta et al, 2012) demonstrated that the volumetric advantage in the brains of men compared with those of women does not appear to have any relation to measurements of overall cognitive acuity. Although there were no differences in the cognitive talent of boys and girls, differences were detected in specific aptitudes: a clear female advantage in verbal reasoning tasks and a considerable male advantage in the spatial rotation of figures. At the level of specific talents, moderate links were shown between the size of cerebral segments (grey and white matter) and intellectual performance, with differences between the sexes. The male advantage in spatial tasks appears to be solid, and is possibly related to them having greater circuitry for simultaneous processing in mapping the configuration of personal space and environment, as explored in tests on the mental rotation of figures or spatial navigation. In contrast, the comparative female advantages in verbal fluency and induction tests and various memory measurements seem to rest on an optimised sequential process (Baron Cohen et al, 2005; Wittelson et al, 2006, Lohman and Lakin, 2009, Andreano and Cahill, 2009). Rooting these differences in cognitive talent, in singular properties of the cerebral structure will, however, require much more precise morphometric examinations and functional scan analysis than the preliminary maps that have been obtained up to now. Studies of sex differences in nuclei, areas and tracts and in cortical and subcortical systems are progressing at a good pace. What is more, despite the fact that variability is inevitable, they are enhancing the pool of robust findings on sexual dimorphisms and their relationship to talents in the human brain.
Resistances to sex differences.
In an essay on the advances made in research into brain dimporhisms in men and women, Larry Cahill suggested, a decade ago, that a series of errors and confusions are often repeated in this issue to the point that they distort the picture enormously. The three main misunderstandings are all linked: 1. there is still a mistaken conviction that the differences between genders are actually small in terms of behavioural and cognitive performance, and their possible neural substrates; 2. these tenuous average distances between both sexes predominantly denote the influence of extreme cases on the distribution of the populations studied; 3. intra-sex differences are usually much greater than inter-sex ones, so the distinctions should be considered trivial. The fourth source of error derives from the consideration that all sex differences, when systematic, respond to the action of sex hormones. This would imply that male and female brains are essentially identical, except for the detail of steroid hormone fluctuations. A final area of confusion results from the mistaken notion that when there is no way of delimiting a sex difference for a specific cognitive performance or attribute, it must be deduced that there will not be differences linked to sex in the neural systems that regulate it (Cahill, 2006).
Although all those notions have been refuted time and again, they persist even among biologists and neuroscientists who work in areas that are far removed from sexual differences. They do not abate, probably because of the ‘controversies’ that come with any inquiry focused on pinpointing differences between genders. In any case, the need to clarify the origins of gender-dependent variability has continued to grow, if only because of the urgency of unravelling the mechanisms behind the considerable disparities in the rate of neurological and psychiatric illnesses between the sexes. This is a considerable hiatus that has long been known. However, some of these anomalies are not trivial conditions of the nervous system that affect huge swathes of the population (painful chronic conditions, depressive and schizophrenic disorders, self-harming behaviour, Alzheimer’s dementia and other neurodegenerative processes, sleep disorders, autism and other developmental genetic anomalies in childhood and adolescence). There has therefore been unparalleled growth in neurochemical and molecular research attempting to pinpoint the different inductors in these syndromes, and potential enhancing factors or protective cascades linked to sex (Berkley 2003, Baron-Cohen et al, 2005, Cahill, 2006). To make full use of the differential targets in the brains of both sexes that molecular genetics are relentlessly opening up, this growth has happened very quickly (Jung Kang et al, 2011). Even the handicaps associated with forms of the ’extreme’ male brain or ‘extreme’ female brain are being dissected (Baron-Cohen et al, 2005, Bremser and Gallup, 2012).
This zealous genetic and neurochemical research into sex differences in normal functioning and psychopathology will also allow a reconnection with areas of psychological research in which sexual differentiation has always been present. Personality is a field in which this was a common focus for exploration: both in statistical approaches to identify ‘universal’ character traits (sociability, neuroticism, aggressiveness/placidity, diligence, appetite for risk and novelty) and in pinpointing temperament attributes that are closer to the clinical threshold (personality disorders, affective disorders) in which sex differences have always played an important role (Gomà 2001; Hyde 2005; Pintzinger et al 2017). Hesitations have been few here: there is a huge abundance of data on inter-sex differences in empathy, affective reactivity, ambition, perfectionism, spirituality, observance of rules, dominance, and sexual permissiveness and promiscuity, to give just a few examples. Whilst, in some of these traits, affective aspects are predominant over cognitive ones, the majority involve confluences of both processes that respond, in turn, to the functioning of circuitry and mediating machinery carrying inter-sex biases. However, it must also be noted that some overall analyses insisted that differences in personality between the sexes are, in fact, small, particularly when the large dimensions of temperament are taken into consideration (Hyde, 2005). Studies of very extensive, representative samples of the US population, which maximised methodological precautions and measured a wide range of personality traits indicate, nonetheless, that there are notable differences in temperament between men and women, approaching the very noticeable hiatus that appears in the different combative profiles and protective and caring behaviour towards offspring (Del Giudice et al, 2012).
From this point on, I am going to take the trait of differential aggression between the sexes as an example because it is, perhaps, the attribute for which the most pronounced disparities have been obtained: men have the upper hand in the use of violent tactics but female aggression displays particular qualities.
Female (tempered) violence.
Women are not benign. Although they know how to lavish affection and self-sacrifice derived from their innate role as carers and protectors, they have combative potential that cannot be ignored. In confrontations between women or in conflicts with men, female aggression unfolds with considerable versatility and efficacy. However, it usually goes more unnoticed because of the intensity and frequency of incidents involving men (Bjorquist et al 1999, Archer 2004, 2009, Tobeña 2001, 2016). It is so obvious that acts of brutality and extreme cruelty are predominantly committed by men that female violence often seems insubstantial or irrelevant as a result, as though women were insufficiently equipped for harmful behaviour. That is not the case.
However, this do not cancel the facts emerging from comparisons of harmful incidents. Nine times out of ten, the perpetrators of homicide, violent physical assault and robbery with menaces are men rather than women. This massive disparity occurs repeatedly in all societies, whether highly civilised or primitive in nature. This applies to both serious incidents and those limited to a minor disputes, altercations or disturbances with no serious consequences. As a result, there is a major difference between the harmful proclivities of men and women respectively, even discounting sexual assaults that are almost exclusively committed by men (Bellencourt et al 1996, Thornhill and Palmer, 2000, Campbell 2006).
The enormity of this difference, however, masks the role of females in harmful aggression. I am not referring to high-profile crimes occasionally carried out by women (female terrorists and gang members, professional torturers, serial killers, adolescents who have tormented female classmates to death or mothers who have inflicted savage harm on their children), as those episodes fall entirely within that 10% of extreme criminal activity. In fact, whilst distorted surveys would appear to indicate a proportion of around 99/1 or even more between the sexes when it comes to harmful proclivities, the actual statistics remain stubbornly at 9/1. On the other hand, the alarming rise in marital homicide rates would sometimes appear to indicate that the number of women murdered by their partners has grown to became an epidemic. Although the perpetrators of fatal acts of domestic violence are predominantly men, the disparity between the sexes is less than might be expected. According to North American statistics for the last two decades, 62% deaths resulting from conjugal violence were caused by husbands and 38% by wives. In Spain, the legal statistics show that 70% of the perpetrators are men and 30% women. So, while the disparity is very great, it is not as massive as you might expect (Magdol et al 1997, Archer 2004, Campbell 2006; Stockley and Campbell 2013).
Types of aggression according to gender
Where ‘low intensity’ aggression in a family context is examined carefully, women generally take the lead in a number of ways Female aggression predominantly manifests itself in the form of rudeness, sarcasm, teasing, insidious acts, negative attitudes and harmful neglect. These findings are very much in line with many others revealing the manifest superiority of females in certain sets of aptitudes and social intelligence. Many females demonstrate this advantage in empathic talent from childhood or early adolescence and use it throughout their adult life. It seems that women’s brains are better equipped than those of men to recognise and interpret the feelings of others and they can, when called upon, use that superiority to harmful effect, resorting to verbal or gestural barbs accurately targeted so as to puncture their adversary’s self-esteem (Kaukianen et al, 1996).
However, in disputes between men and women, this difference in ability must be offset against the disproportion in physical strength between the sexes. On average, women have a body mass 10-20% less than men, which can result in a 50% disadvantage in terms of muscular strength. This is a massive handicap that has probably played a determining role in the division of tasks and balance of power from our ancestors onwards. It is, however, less of a consideration these days thanks to the progress of moral values based on non-aggression that is largely the result of technological advances (see the illustrative data sets in Pinker, 2011). At the same time, this disparity in physical strength remains relevant because it affords a telling advantage in serious disputes. That is why men more often resort to physical violence while women use other harmful tactics. However, in disputes between women, while subtle aggression can take many forms, it is not unusual for it to escalate into a physical attack (hooliganism, for example is common among female football players and fans, and in other sporting activities).
It can therefore be seen that there are substantial differences between male and female aggression. Men opt for tactics involving physical violence much more frequently than women. The mechanism that supports this difference is the difference between the physical make-up and neuroendocrine machinery of the competitive drive in both sexes. By contrast when it comes to tactics that involve verbal, gestural or indirect aggression (aimed at the interests, status or reputation of rivals), men and women display very similar skills, while in certain strategies linked to social cognition, women clearly do better than men (Archer 2004, 2009; Campbell 2006, Benenson et al, 2011, Stockley and Campbell 2013, Tobeña 2016).
Distinctive combative brains?
These differences in aggression levels cannot currently be attributed to gender differences regarding the circuits that form an integral part of the brain’s readiness to attack or defense. As far as we know, the neural regions that orchestrate physiological and behavioural aggression do not differ distinguishably between the sexes (Niehoff, 1999, Nelson and Trainor 2007). However, they are structures from the most ancient machinery of the affective brain. They are difficult to access and have still not been tackled with the highest resolution scanning or zonal neuromorphology techniques, which are already being applied in rodents and apes. Nevertheless, there are clear gender differences in some subcortical systems (amygdala, hypothalamus) that orchestrate reactions of emotional aggression such as rage or hostility. This has even led the higher level of anti-social personality disorders in men being associated with a lower amount of grey matter in individuals harbouring these potentially violent psychopathies in certain ventral, medial and orbital regions of the pre-frontal cortex. Figures are emerging that corroborate this nexus between zonal deficits in pre-frontal grey matter and that differential association of psychopathy between sexes, in normative samples without any contact with the legal or clinical system (Raine et al, 2011).
One place where there are clear sex differences is in the neuroendocrine arsenal that acts on these regions of the brain to modulate their work. By that, I am referring to the substances that come from the peripheral body or are produced in the brain itself and act on these neural zones to enable or inhibit the competitive drive or aggressive outbursts. Sex hormones are the leading protagonists here. Androgens act as enablers of combativeness and dominance, and the available figures are very different in men and women, and vary enormously within each sex. There are already robust data confirming that women prone to physical pugnacity, dominance and leisure activities with a high physical risk present unusual androgenic figures (Dabbs et 1988, Pajer et al, 1998, 2001; Maras et al, 2003). In other words, females with a more ambitious and bold temperament have an androgynous bias, despite the absence of testicular glands. On the other hand, the hormonal variations that are typical of the menstrual cycle generate changing reactivity in mood, which is accompanied by variations in the threshold of irritability in response to mishaps. And it doesn’t end there, of course. Many other substances modulate aggressive outputs by promoting or inhibiting combativeness (Pfaff 2011).
Alarm or stress hormones (those in the corticosteroid axis: adrenaline, noradrenaline, vasopressin) play a very important role in competitive confrontations and differences between the sexes are consistent. For example, some data indicate that female adolescents who repeatedly demonstrate disruptive behaviour not only share higher testosterone profiles with more rebellious boys, they also have consistently low levels of cortisol (the target hormone in harmful stress). Sex differences in the central serotonergic or opioid function are being precisely mapped and influence the competitive styles of both sexes, as there are plenty of findings relating them to inhibitory modulation of aggression. And there is more. Oxytocin levels modulate closeness, enjoyment, trust and affective dependence, and that normally involves a restriction of aggressive expression. Data is also beginning to emerge showing that central vasopressin induces aggression related to possessive jealousy in animals that form pair bonds, and in humans. Jealousy is an emotional driver with distinctive traits depending on gender, and is very common in serious sentimental conflicts. The work to outline neuroendocrine profiles that support the different aggressive traits in men and women of course goes much further than this limited summary suggests (Niehoff 1999, Pfaff 2011).
Pugnacity and cultural environment.
In the end, does everything depend on the prescriptions and modulations of biological command? Should the differences in combative temperament and mood between men and women be attributed to a detailed description of the neuroendocrine cascades and the neural circuitry of aggression? Where do the influences of patriarchal culture, different educational styles, inequalities and discriminatory ‘glass ceilings’ within ‘egalitarian’ societies come in? Well, the message is this: sex differences in pugnacity must be embedded, at the very first, in the neurochemical machinery, as there is no sharper division than sex in biological differentiation. Not only is an entire chromosome involved, but also a vast variety of molecular signals is devoted to shaping external morphologies and internal devices that are abundantly different. This includes the brain, where the expression of a character trait as conspicuous as aggressiveness is ultimately concocted (Valla et al, 2011).
I have reviewed enough data to be able to corroborate the fact that women are not benign. They are not, although it has been proved time and again that their combative styles cause much fewer deaths than those of their male counterparts. Nevertheless, cultural influences have an inescapable influence as they can alter this differential morbidity. I can give two examples. Recently, a slight but definite upward trend in female combativeness has been seen on all fronts, and this is eroding the ominous 9/1 disproportion of male compared with female harm. These are tentative findings, but in aggression that leads to imprisonment, and less significant clashes and disturbances, this disproportion shrinks to nearer 8/2. This growing female presence in harmful combativeness must be attributed, in principle, to recent cultural variations that have enabled a malignancy inhibited by social constraints to emerge (Magdol et al, 1997). There are other supporting data: in very disruptive female adolescents (with frequent episodes of truancy, running away from home, shoplifting, vandalism, fighting with peers, or cruelty to animals or weaker people), the differences between boys and girls are less than expected. The average rate of this behavioural anomaly is 8% for girls and 12% for boys. This combative hiatus is therefore much more modest. A large proportion of these female adolescents achieve satisfactory socialisation in subsequent years, but a negligible portion of them continue with their disruptive behaviour into young adulthood and their adult years.
The following example concerns the influence that the number of hours of television watched during childhood, adolescence and young adulthood has on subsequent aggressive behaviour. The most robust evidence comes from a longitudinal study of a representative sample of families in the State of New York (Johnson et al, 2002). Over a period of 25 years, it assessed the male and female children in 707 mostly white Catholic families, who were five years old when monitoring began. A range of data was collected on four different occasions over the period, and the final assessment in the year 2000 included not only the questionnaire responses of children and mothers, but data from FBI police records. The results indicated that high consumption of television during adolescence (at age 14) was linked to a higher rate of aggressive behaviour at 16 and 22, with the highest figures found in boys. This connection was seen again when relating the hours spent watching television at age 22 with aggressive behaviour measured at age 30, but in this case the effect was much stronger in girls, to the point that the results were equal to those for men. Overall, these findings revealed that there is a subgroup of young people of both sexes in whom a high level of television consumption coincides with all kinds of disruptive and criminal behaviours, without it being possible to discern a clear direction in this self-perpetuating cocktail (aggressive temperament + high consumption of television). It must be said that this effect also continued when the influence of a low socio-economic level, childhood abandonment, early abuse, consumption of illegal substances, and other contextual variables that predict aggression, was excluded.
Conclusion: feminine and masculine power.
It must be stressed that research into sexual dimorphisms in humans has already progressed from the descriptive, correlational stage to targeted experiments. Women administered with low doses of testosterone lose their capacity for emotional recognition and aptitudes related to cognitive empathy. That is to say, their advantage in nuclear attributes of social intelligence is reduced, where they usually far exceed men. The same happens with hostility, risk aversion and fear of physical threats, in line with functional changes in the workings of the cerebral circuitry that modulates aggression (Van Honk et al, 2011). It has also been demonstrated that female caution in risky financial decisions can be altered with an androgen boost (Sapienza et al, 2009). Overall, it has been proved that an induced neuroendocrine bias that acts temporarily on distinctive, key machinery can change the cognitive style and affective characteristics of one of the genders, making them more similar to the opposite sex.
This all reinforces the approach I have taken here and authoritatively illustrates an assertion supported by much of the data: men and women have neural circuits and neuroendocrine arsenals to help them cooperate and compete valiantly in very complex and ever-changing social scenarios. They are undoubtedly the most complex in the entire animal kingdom. On both sides, their performance is outstanding and generally similar, with some optimised specialisations and profiles. Ultimately, men and women use brains and neurochemical cocktails that are partly different, in order to reach demanding benchmarks that are, as far as possible, similar in all contexts of human struggles, including battles for social power (Tobeña, 2008). It is the task of neuroscientists to reveal the different and common routes in both genders without becoming overly contaminated by the noise of incessant confrontations intended to achieve social and political preeminence or parity (Stoet and Geary 2015; Stern, 2016).
[1] There are continuing controversies about subtle cultural factors that could lead to different ceilings in some disciplines, and these have led to some thought-provoking exchanges. See, for example, the conflicting data from Ginter and Khan (2015) and Ciampani and Leslie (2015) on the effect of expectations in relation to differential natural talent.
Referencias.
- Allen JS, Damasio H., Grabowski TJ, Bruss J. and Zhang W (2003) Sexual dimorphism and asymmetries in the gray–white composition of the human cerebrum, Neuroimage 18, 880–894 (2003).
- Andreano JM and Cahill L (2009) Sex influences on the neurobiology of learning and memory, Learning andMemory, 16, 248-266.
- Archer, J. (2004). Sex differences in aggression in real-world settings: a meta-analytic review. Review of General Psychology, 8, 291–322.
- Archer J (2009) Does sexual selection explain human sex differences in agression?, Behavioral and Brain Sciences, 32, 249-311.
- Baron-Cohen S, Knickmeyer RC and Belmonte MK (2005) Sex differences in the brain: implications for explaining autism, Science, 310, 819-823.
- Benenson JF, Markovits H, Thompson ME and Wrangham RW (2011) Under threat of social exclusion, females exclude more than males, Psychological Science, 22, 4, 538-544
- Berkley KJ (2003) Sexual differences in pain, Behavioral and Brain Sciences, 20, 371-380.
- Bettencourt BA and Miller N (1996) Gender differences in aggression as a function of provocation: a meta-analysis, Psychological Bulletin, 119, 422-447.
- Bjorquist K, Osterman K, Kaukiainen A and Logerspetz KMJ (1999) Concomitants of physical, verbal and indirect aggression, Aggressive Behavior, 25, 1, 35.
- Bremser JA and Gallup GG (2012) From one extreme to the other: negative evaluation anxiety and disordered eating as candidates for the extreme female brain, Evolutionary Psychology, 10, 457-486.
- Burgaleta M, Head K, Alvarez-Linera J, Martínez K,Escorial S, Haier R and Colom R (2012) Sex differences in brain volume are related to specific skills, not to general intelligence, Intelligence, 40, 60-68.
- Cahill L (2006) Why sex matters for neuroscience, Nature Reviews Neuroscience, 7, 6, 477-484.
- Campbell A (1999) Staying alive: evolution, culture and women’s intra-sexual aggression, Behavioral and Brain Sciences, 22, 203-252.
- Ceci SJ and Williams WM (2010a) The mathematics of sex: how biology and society conspire to limit talented women and girls, New York: Oxford University Press.
- Ceci SJ. And Williams WM (2010b) Sex differences in math-intensive fields, Current Directions in Psychological Science, 19, 275–279.
- Ciampiani A and Leslie SJ (2015) Response to comment on expectations of brilliance underlie gender distributions across academic disciplines, Science, 349, 6246, 391-c.
- Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks , Clark ChA and
Vargha-Khadem F (2012) Normative Development of White Matter Tracts: Similarities and Differences in Relation to Age, Gender, and Intelligence, Cerebral Cortex, 22, 8, 1738-1747.
- Dabbs JM, Barry Ruback R, Frady RL, Hopper ChH and Sgoutas DS (1988) Saliva testosterone and criminal violence among women, Personality and Individual Differences, 9, 2, 269-275.
- Del Giudice M, Booth T and Irving P (2012) The distance between Mars and Venus: measuring global sex differences in personality, PLOsOne, 7, 1, e29265.
- Escorial S, Román FJ, Martínez K, Burgaleta M, Karama Sh and Colom R (2015) Sex differences in neocortical structure and cognitive performance: a surface-based morphometry study, Neuroimage, 104, 355-365.
- Georgiadis JR, Kringelbach LM and Pfaus JG (2012) Sex for fun: a synthesis of human and animal neurobiology, Nature Reviews Urology, 9, 486-498.
- Ginter DK and Khan Sh (2015) Comment on Expectations of brilliance underlie gender distributions across academic disciplines, Science, 349, 6246, 391-b.
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV and Tsuang MT (2001) Normal sexual dimorphism of the adult human brain as assessed by in vivo magnetic resonance imaging, Cerebral Cortex, 11, 490-497.
- Gomà M (2001) Prosocial and antisocial aspects of personality in women: a replication study, Personality and Individual differences, 30, 1401-1411.
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P and Gur RE (1999) Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance, The Journal of Neuroscience, 19, 10, 4065-4072.
- Haier RJ, Jung RE, Yeo RA, Head K and Alkired MT (2005) The neuroanatomy of general intelligence: sex matters, Neuroimage, 25, 320-327.
- Hoffman M, Gneezy U and List JA (2011) Nurture affects gender differences in spatial abbilities, PNAS, 108, 36, 14786-14788.
- Hyde JS (2005) The gender similarities hypothesis, American Psychologist, 60, 581–592.
- Hyde JS and Mertz JE (2009) Gender, culture and mathematics performance, Proceedings of the National Academy of Sciences USA, 106, 8801–8807
- Johnson JG, Cohen P, Smailes EM, Kasen, S and Brook J (2002) Television viewing and aggressive behavior duing adolescence and adulthood, Science, 295, 2468-2471.
- Jones CM, Braithwaite VA, Healy SD (2003) The evolution of sex differences in spatial ability, Behavioral Neuroscience, 117, 403-411.
- Jung Kang H et al (2011) Spatio-temporal transcriptome of the human brain, Nature, 478, 483-489.
- Kaukiainen A, Bjorkquist K, Osterman K and Lagerspetyz KMJ (1996) Social intelligence and empathy as antecedents of different types of aggression, in CG Ferris, and Th Grisso (Eds) Understanding aggressive behavior in children, Annals New York Academy of Sciences, Vol. 794, 364-66.
- Kimura D (2000) Sex and cognition Cambridge, MA: MIT Press.
- Lenroot RK, Gogtay N, Greenstein DK,Wells EM, Wallace GL,Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM and Giedd JN (2007) Sexual dimorphism of brain developmental trajectories during childhood and adolescence, Neuroimage, 36, 1065-1073.
- Lohman D and Lakin J (2009) Consistencies in sex differences on the cognitive abilities test across countries, grades, test forms and cohorts, British Journal of Educational Psychology, 79, 389–407
37. Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H and Toga AW (2004) Gender differences in cortical complexity, Nature Neuroscience, 7, 799 – 800
- Magdol L, Moffit TE, Caspi A, Newman DL, Fagan J and Silva PA (1997) Gender differences in partner violence in a birth cohort of 21-year-olds: bridging the gap between clinical and epidemiological approaches, Journal of Counsulting and Clinical Psychology, 65, 68-78.
- Maras A, Laucht M, Gerdes D, Wilhelm C, Lewicka S, Haack D, Malisova L and Schmidt MH (2003) Association of testosterone and dihydrotestosterone with externalizing behavior in adolescent boys and girls, Psychoneuroendocrinology, 28, 932–940.
- Nelson D and Brammer C (2010) A national analysis of minorities in science and engineering faculties at research universities (http://chem.ou.edu/*djn/diversity/faculty)
- Nelson RJ and Trainor BC (2007) Neural mechanisms of aggression, Nature Reviews Neuroscience, 8, 536-546.
- Niehoff D (1999) The biology of violence: how understanding the brain, behavior and environment can break the vicious cycle of aggression, New York: Free Press.
- Pajer KA (1998) What happens to “bad” girls?: a review of the adult outcomes of antisocial adolescent girls, American Journal of Psychiatry, 155, 7, 862-870.
- Pajer K, Gardner W, Rubin RT, Perel J and Neal S (2001) Decreased cortisol levels in adolescent girls with conduct disorders, Archives of General Psychiatry, 58, 3, 297-302.
- Pfaff DA (2011) Man and woman: an inside sotory, NY: Oxford University Press.
- Pintzinger NM, Pfabigan DM, Pfau L, Kryspin-Exner I and Lamm C (2017) Temperament differentially influences early information processing in men and women: preliminary electrophysiological evidence of attentional biases in healthy individuals, Biological Psychology, 122, 69-79.
- Pope HJ, Kouri EM and Hudson JI (2000) Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial, Archives of General Psychiatry, 57, 2, 133-140.
- Pinker S (2011) The better angels of our nature: why violence has declined, N.Y.: Viking Penguin.
- Raine A, Young Y, Narr KL and Toga W (2011) Sex differences in orbitofrontal gray as a partial explanation for sex differences in antisocial personality, Molecular Psychiatry, 16, 227-236.
- Sapienza P, Zingales L and Maestripieri D (2009) Gender differences in financial risk aversion and career choices are affected by testosterone, PNAS, 106, 15268–15273.
- Shah NM, Pisapia DJ, Maniatis, S Mendelsohn MM, Nemes A and Axel R (2004) Visualizing sexual dimorphism in the brain, Neuron, 43, 313–319
- Sommer IE, Aleman A, Bouma A, Kahn RS (2004) Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies, Brain, 127, 1845–1852.
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM and Toga AW (2007) Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age, Cerebral Cortex, 17, 1550-1560.
- Stern Ch (2016) Undoing insularity: a small study of gender sociology’s big problem, Econ Journal Watch, 13, 3, 452-466.
- Stockley P and Campbell A (2013) Female competition and aggression: interdisciplinary perspectives, Philosophical Transections of the Royal Society-B, 368, 20130073.
- Stoet G and GearyDC (2015) Sex differences in academic achievement are not related to political, economical or social equality, Intelligence, 48, 137-151.
- Thornhill R and Palmer CT (2000) Why men rape, The Sciences, 1, 30-36.
- Tobeña A (2001) Anatomia de la agresividad humana, Barcelona: Galaxia Gutenberg.
- Tobeña A (2006) El cerebro erótico, Madrid: La Esfera de los Libros.
- Tobeña A (2008) Cerebro y poder, Madrid. La Esfera de los Libros.
- Tobeña A (2016) Neurologia de la maldad, Barcelona: Plataforma.
- Valla JM and Ceci SJ (2011) Can sex differences in science be tied to the long reach of prenatal hormones? Brain organization theory, digit ratio (2D/4D) and sex differences in preferences and cognition, Perspectives on Psychological Science, 6, 2, 134-146.
- Van Honk J, Schutter DJ, Bosa PA, Kruijtc AW, Lentjesd EG and Baron-Cohen S (2011) Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio, PNAS, 108, 8, 3448-3452.
- Wittelson S, Beresh H and Kigar DL (2006) Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors, Brain, 129, 386-398.
[1] Las polémicas sobre sutiles factores culturales que podrían conducir a techos diferenciales en algunos ámbitos disciplinares no cesan (27) y han dado lugar a intercambios sugerentes. Véase, por ejemplo, los datos confrontados de (16) y (22), sobre el efecto de las expectativas en cuanto al talento natural diferencial.

Adolf Tobeña
Monographic Giflted women, fragile men
Departamento de Psiquiatria y Medicina Legal. Instituto de Neurociencias. Universidad Autónoma de Barcelona
March 2017
